Home
Silver stain
Coomassie blue stain
SDS PAGE graphics
SDS PAGE basics
Gel electrophoresis tips
|
SDS gel electrophoresis - advice for beginners
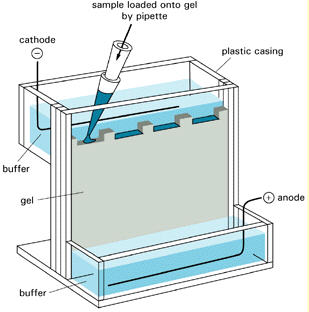

Buffers
Electrophoresis is generally more sensitive to salt concentration than to pH. Adjusting buffers near their pKa with a pH meter is risky. Small pH changes lead to moderate changes in salt concentration. For electrophoretic buffers (near the pKa) it's best to weigh and pipet (i.e. 12N HCl) components rather than adjusting with a pH meter. I find the conductivity meter a more reliable tool for characterizing buffers than the pH meter.
Completing the electrical circuit
Make sure the electrical path is clear between anode and cathode wells. Remove bubbles from the bottom of the gel.
For reproducible electrophoresis
When you adjust the voltage on your power supply note the current. If the current differs from last time, then some component of the system (likely buffer composition) has changed.
Laemmli separating gel buffer
Buffer chloride concentration (not pH) greatly affects separation. High chloride improves stacking, but smaller proteins run with the front. Low chloride facilitates separation of small proteins at the expense of stacking. Adding HCl volumetrically from reagent con HCl is simpler and more reliable than adjusting with a pH meter.
Sample buffer - tips for preparing samples
Sample (cracking) buffer has little effect on separation. pH 6.8 Tris buffer is very poor for reducing proteins (the pH is about 4.5 at 100oC!). Choose a buffer with a higher pH at 100oC.
Quality of SDS
Electrophoresis grade SDS is synthesized from purified dodecyl alcohol. Lower grades generally employ alcohols from palm oil (which contains longer chain alcohols). 95% SDS generally means 95% alkyl sulfates (not that 95% of alkyl chains are C12.) Either grade will work, but gel mobilities aren't strictly comparable (i.e. don't take MWs too literally - which you shouldn't anyway).
Older SDS
As long as stock solutions are soluble your SDS is OK. If they're insoluble the SDS is partially hydrolyzed (by moisture).
How much SDS do I need?
0.1% SDS is standard for running gels. This is plenty, provided that your proteins are already SDS complexes. Be aware that proteins bind about twice their weight in SDS so that you may need much more SDS in your sample buffer to saturate the proteins. If your sample contains lipids you'll need still more SDS. If in doubt add more SDS to your sample and see if it improves separation.
Reducing agents
Proteins are normally reduced (SS bonds broken) with a thiol in the sample buffer before electrophoresis. Mercaptoethanol (ME) is the standard reducing agent. Since it's a relatively weak reducing agent it's required in large amounts. Both ME and reduced proteins reoxidize with time. Freezing does not inhibit reoxidation. I prefer to reduce with dithiothreitol (DTT, a stronger reducing agent) and then alkylate proteins with iodoacetamide to stabilize them in the unfolded state.
Advice on samples
Start with simple, well characterized samples - for example purified proteins, human serum, bovine serum, etc. Move on to complex and/or "nasty" samples only when the technique is working well. I find phenol extraction a good way to eliminate junk from proteins
For a brief review of electrophoresis:
Lane, LC (2001) Electrophoresis. Pp 394-396 in Encyclopedia of Plant Pathology. Ed Maloy, OC and Murray, TD. Wiley-Interscience (New York)