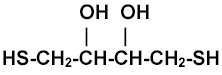
Stabilizing and "resurrecting" DTT stock solutions
Ensure that your DTT is fully reduced
I run many SDS-protein gels and stain them with silver. I reduce and alkylate samples to prevent " rain" (see reduction-alklyation bibliography). Mercaptoethanol is inadequate since it's a weak reductant and would be required in large volumes. Dithiothreitol (M=154.3) reduces proteins at millimolar levels requiring only a slight excess of iodoacetamide (or N-ethylmaleimide) to alkylate. I've found DTT sometimes unsatisfactory because it's partially (perhaps mostly) oxidized. This manifests itself as smeary bands on the gel, particularly for large, cys rich proteins such as BSA. Oxidized DTT absorbs weakly (340 nm) so oxidation can be monitored, but this is inconvenient. It's more convenient to reduce DTT with tributylphosphine (TBP).
Procedure
To 0.4 ml of 0.5 M (1.0 N) DTT (30mg) in a small conical plastic vial add 100 ul of tributylphosphine (TBP) and 100 ul of 1-bromo-3-chloropropane. Mix. Withdraw aliquots from the upper phase to reduce protein samples.
Principle
TBP (cheap) is a stronger reducing agent that DTT and reduces oxidized DTT. TBP is less dense than water, 1-bromo-3-chloropropane (or other dense organic solvent) inverts phases, putting DTT on top for convenience.
Why not just freeze the solution?
Oxygen solubility increases as temperature decreases. Freezing creates cracks which facilitate oxidation.
Oxygen
Oxygen concentration in air is about 10mM. Solubility in water is of the order of 0.2mM. Maintaining thiol solutions in the reduced state is engineering. Keep the thiol as concentrated as possible and oxygen volume as low as possible.
"Good" and "bad" DTT compared.