Cellulose, cellulase
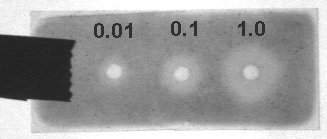
Wells were cut in 0.4% agarose gels (on GelBond) containing 1.0% carboxymethylcellulose. Aspergillus nidulans cellulase (5mg/ml plus dilutions) was introduced into wells and incubated for 3 hrs at 45C. One set was stained with Congo red (0.1%) and another with toluidine blue O (0.05%) The latter was destained overnight with DW. Both were photographed with the BioRad ChemiDoc through the orange filter. The Congo red wasn't visible and was therefore acidified with 1% HCl to convert it to the more photogenic blue form.
Toludine Blue O
Congo Red (acidified)
Alcian blue staining was less successful. The dye was unstable (ppt.) in DW. In 1% HOAc it remained fairly soluble, but left a blue ppt on the gel surface which destaining did not remove.
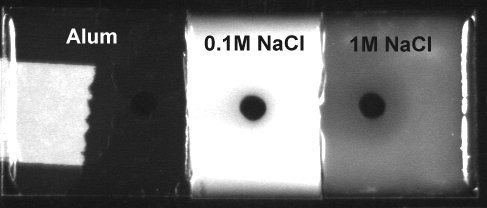
*****Attempt to stain CMCcellulose with cetyltrimethylammonium bromide (CTAB). Aspergillus nidulans cellulase (5mg/ml) was introduced into wells and incubated 3hrs at 45C. The Gelbond was cut into 3 pieces and each was incubated overnight with 0.2% CTAB containing NaCl (as specified on photo). Photographed with the BioRad ChemiDoc with epi-illumination and w/o filter
Conclusions - high salt reduces ppt. The area around wells (hydrolyzed CMCcellulose) often shows stronger ppt than the gel as whole. This could reflect "staining" of protein or hydrolyzed CMCcellulose.
*****A similar experiment with polyethyleneimine (0.2%) in place of CTAB gave similar results. The PEI predipitates were more resistant to dissolution by salt. Ammonium alum (0.1M) did not ppt CMCcellulose. (below) Cationic (zwitterionic) dyes remain the most promising detection agents.
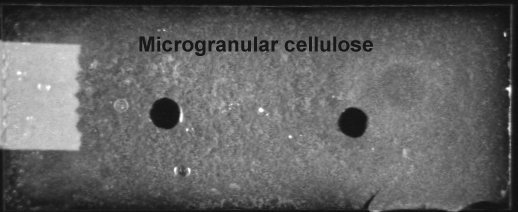
Aspergillus nidulans cellulase (5mg/ml) overnight at 45C did not visibly affect microgranular cellulose (below)
*****