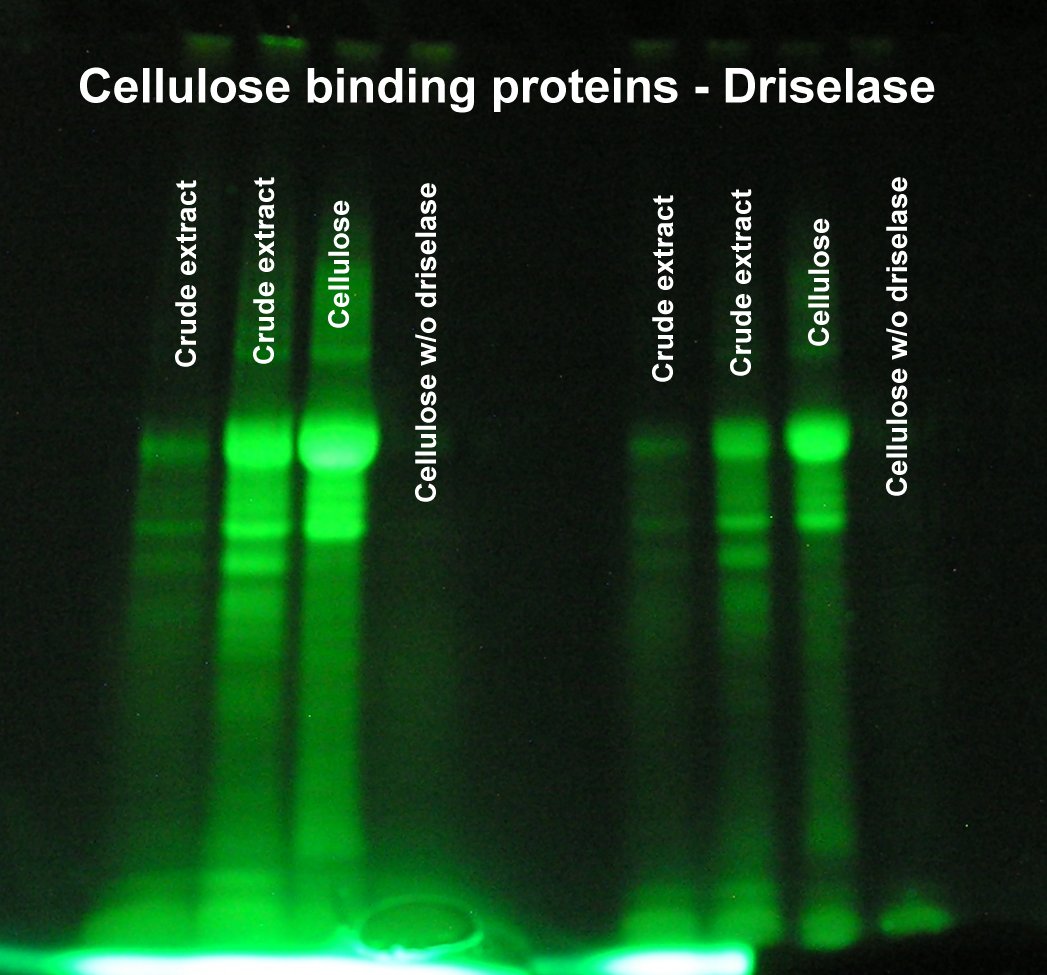
Isolation of cellulose binding proteins
Driselase was chosen as starting material since the methylumbelliferyl assay showed considerable active cellulase. 300 mg of Driselase was dissolved in 10 ml of 0.2M sodium citrate, pH 5. The resulting suspension was centrifuged yielding a large pellet and a brownish supernatant. 2.0 g of microgranular cellulose was suspended in the solution and mixed for about 10 min. The suspension was centrifuged 5 min at 5K in a 30 ml Corex tube. The (brownish) supernatant was discarded. The pellet was washed 4x by suspending in 20 ml of 0.2M pH 5 sodium citrate and centrifuging 5min at 5K. The final pellet was suspended in 10 ml of citrate buffer and extracted with 4 ml of phenol. The phenol (lower) layer was transferred to a fresh 30 ml Corex tube and precipitated with 20 ml of acetone. Precipitation was extensive. About 1.5 ml of the suspension was transferred to a 15 ml Corex tube and centrifuged 5min at 7K. The (large) pellet was washed with acetone, dried and suspended in 350 ul of borate cracking buffer. A parallel cellulose sample washed in the same way, but w/o added enzyme gave no visible precipitate and was suspended in 75 ul of cracking buffer, reduced and alkylated, and modified with dansyl chloride.
A sample of the clarified supernatant of the original driselase sample was phenol extracted, acetone precipitated, reduced and alkylated, and dansylated similarly.
MW markers were not included, but the major band is clearly a large (~60K) protein. The crude driselase is surprisingly "pure" considering the its purported glycosylase activities. The cellulose binding assay removed most of the brown color and at least one significant protein (compared to the crude extract). The large ppt volume suggests that 25 to 50 mg of cellulose should suffice for future binding assays.
The binding assay (affinity ppt) is clearly useful for identifying cellulose binding proteins in crude extracts.